ANTIRETROVIRAL RESISTANCE

ANTIRETROVIRAL THERAPY
In the infective cycle of HIV, there are several crucial steps targeted by the design of antiretroviral drugs to inhibit virus replication. Among these steps, key enzymes include reverse transcriptase (RT), responsible for converting viral RNA to DNA during transcription; protease (PR), involved in virion maturation and infective virus formation; and integrase (IN), which facilitates the integration of viral DNA into the host genome.
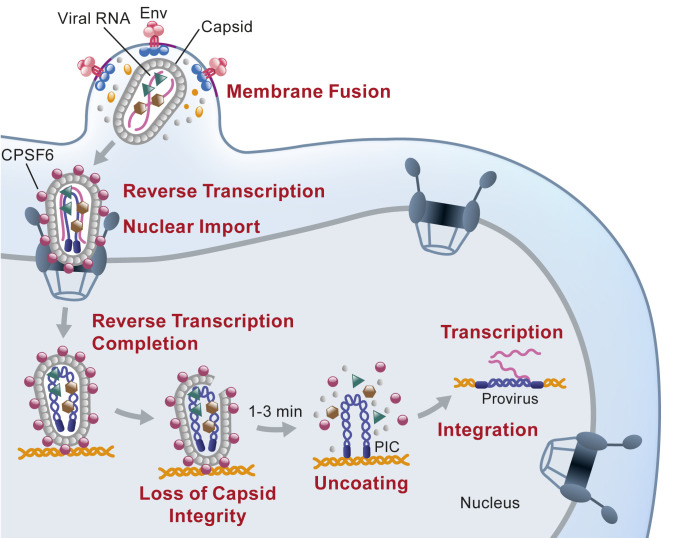
HIV cycle. Li C, et al. doi: 10.1073/pnas.2019467118.
These three enzymes are the molecular targets of the main families of antiretroviral drugs. One family consists of reverse transcriptase inhibitors that specifically target HIV-1 RT and can be nucleotide analogs (NRTI) and non-nucleotide reverse transcriptase inhibitors (NNRTI). Other families include protease inhibitors (PI) and integrase inhibitors (INI). The triple combination of 2 NRTIs plus a third drug (NNRTI, PI, or INI) forms the basis of current antiretroviral therapy. Other available drugs include entry inhibitors, fusion inhibitors, and new maturation inhibitors, among others, which are combined with other "classic" antiretroviral drugs.
Main families of antiretroviral drugs
GOALS OF ANTIRETROVIRAL THERAPY
As of today, there is still no cure for HIV that completely eradicates the virus. Antiretroviral treatment can only reduce the viral load in the body by inhibiting viral replication. However, it cannot prevent the virus from remaining latent within the genetic material (DNA) of infected cells (cellular reservoir) or in specific anatomical sites (anatomic reservoirs) where drugs may not effectively reach, such as the brain.
Nevertheless, by decreasing the viral load, antiretroviral therapy helps preserve immune system function by preventing HIV from destroying vital immune cells. Consequently, this reduces HIV-related morbidity and prevents progression to AIDS, ultimately enhancing the quality of life for those living with the infection.
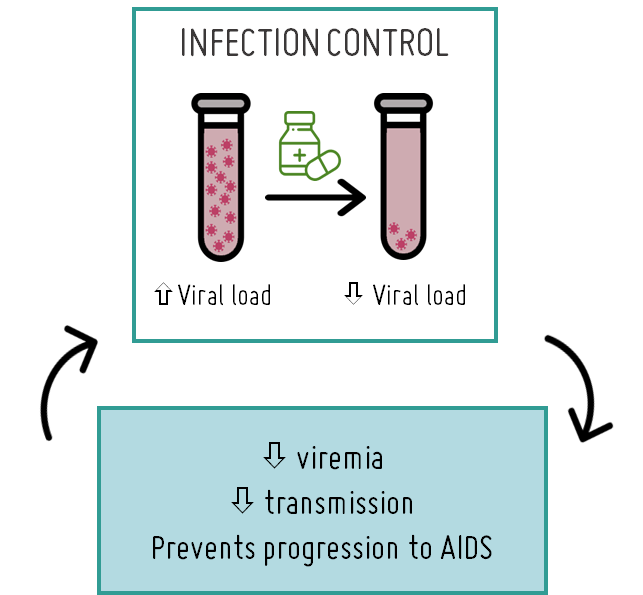

To achieve these goals, it is crucial to initiate treatment promptly (early treatment) and adhere closely to the prescribed antiretroviral regimen (good adherence). These factors heavily rely on adequate healthcare coverage, facilitating early HIV diagnosis and ensuring regular clinical monitoring of the patient's immunological status. This allows for assessing treatment effectiveness in reducing viral load and determining if treatment failure is attributed to the emergence of drug-resistant strains. The World Health Organization recommends conducting resistance testing in the infected population before initiating therapy to identify patients with resistant strains and regularly monitoring treated individuals to identify cases of treatment failure due to the selection of resistant viruses.
FAILURE OF ANTIRETROVIRAL TREATMENT
Therapeutic failure resulting from resistance to antiretroviral drugs, or virological failure, is characterized by incomplete viral suppression with detectable viral load or persistent rebounds after previously achieving an undetectable viral load. As current treatments only achieve low levels of viral suppression rather than complete eradication, there is a risk that drugs may select for HIV variants carrying mutations that reduce the virus's susceptibility to one or more antiretroviral drugs included in the regimen or render it resistant to them. Resistance mutations (DRM) can also arise due to poor treatment adherence, inappropriate dosing regimens, or inadequate drug levels. To mitigate the selection of resistance mutations, antiretroviral drugs are typically used in combination therapy, and the treatment plan may be revised if failure is detected, with affected antiretrovirals being replaced by others still effective in suppressing viral replication.
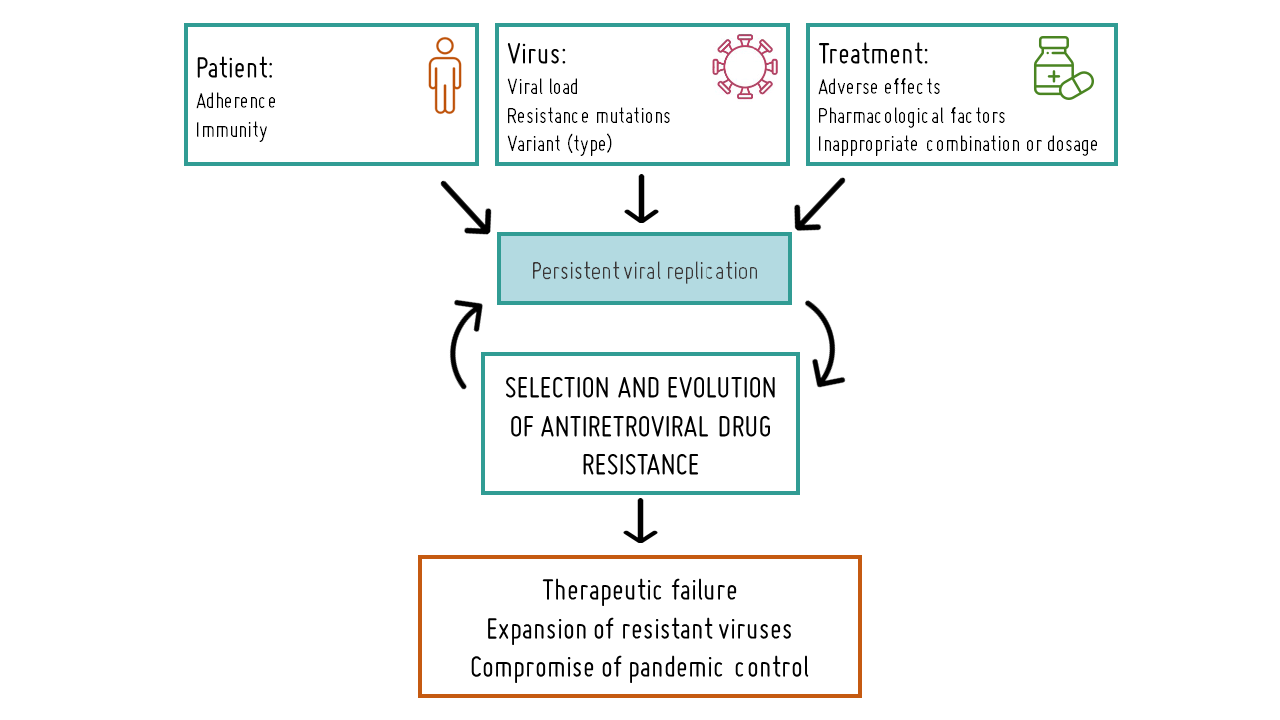
Selection of resistance in HIV
RESISTANCE MUTATIONS
Resistance mutations refer to alterations in specific regions of the virus genome, primarily in areas encoding the target proteins of antiretroviral drugs. These alterations diminish the drug's efficacy through various mechanisms. However, not all resistance mutations hold equal significance. There are primary or major mutations that profoundly affect susceptibility to one or more drugs, defining the virus's pharmacological resistance and potentially reducing its replicative capacity. Secondary or accesory mutations have a lesser impact on resistance but can still modulate it by boosting viral replication, with some occurring naturally in certain HIV variants. Resistance mutations can also be transmitted when they appear in patients who have never received antiretroviral treatment (naïve patients) or acquired, which are those selected in patients who have received antiretroviral treatment.
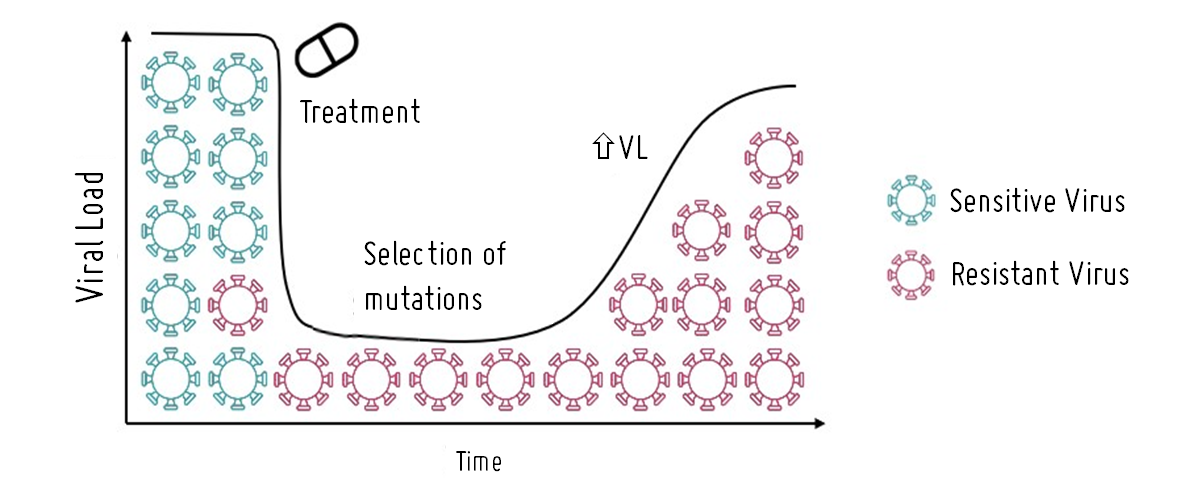
Selection of resistances in HIV
At the clinical level, genotypic techniques are employed to detect DRM, involving the amplification of regions of the HIV genome encoding viral proteins targeted by antiretrovirals (typically protease, reverse transcriptase, and integrase) through PCR to obtain their sequences. These sequences are then analyzed to identify mutations and infer their impact on susceptibility to each of the antiretroviral drugs in therapeutic use, often through specialized websites.
Following the identification of mutations and their impact on drug susceptibility, adjustments can be made to the patient's treatment regimen by eliminating the most affected drugs, thereby ensuring treatment effectiveness and preventing an increase in viral load and associated health consequences.

PROJECTS ON ANTIRETROVIRAL DRUG RESISTANCE
At EPIMOLVIH, we have been dedicated to studying resistance mutations to antiretroviral drugs in cohorts of children and adults with HIV from Spain and from countries with limited resources in Africa and Latin America for several years. In these regions, resistance monitoring is often insufficient or absent due to logistical and economic constraints. Our work involves identifying mutations and assessing their impact on susceptibility to each drug, as well as their natural presence across different HIV variants (types, groups, subtypes, and CRF recombinants). We collaborate closely with local clinicians, providing them with information about the resistances observed in their patients included in our studies. This enables them to make informed therapeutic decisions and switch to more effective drugs when necessary. Additionally, our findings are disseminated through publication in international journals, informing policymakers and contributing to the adaptation of therapeutic guidelines to address emerging resistance patterns..
Given the critical importance of this research, the study of drug resistance mutations is integrated into the majority of our projects. You can find our studies on HIV antiretroviral drug resistance in various scientific publications and doctoral theses.
Would you like to collaborate with us in advancing research on HIV treatment resistance?
You too can contribute your bit to continue advancing research on antiretroviral drug resistance in HIV and improve treatment regimens in resource-limited countries.
Would you like to collaborate?
Join our Teaming group for just one euro per month or donate whatever you consider appropriate as an individual or company.
Together for the advancement of research on antiretroviral drug resistance in HIV!



